COVID-19 vaccine to be made available for individuals 16 and older in DuPage County on April 12
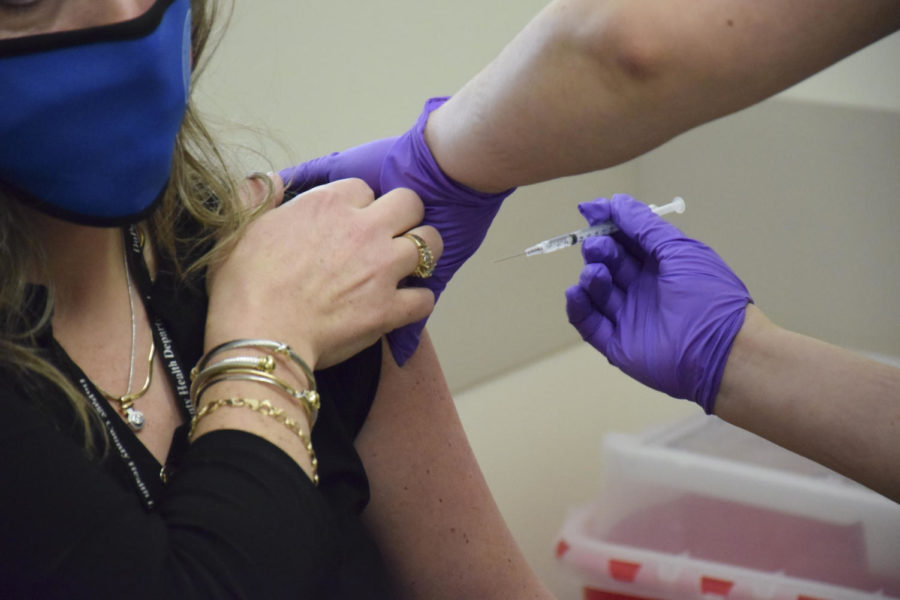
Photo courtesy of Stephanie Calvillo
A nurse at the DuPage County Health Department prepares to vaccinate a department safety officer.
April 9, 2021
DuPage County is expected to reach Phase 2 of its COVID-19 vaccine rollout by April 12, allowing everyone 16 or older to receive the shots. Currently, only the Pfizer vaccine has been approved for individuals 16-18, whereas adults can receive the Pfizer, Moderna or Johnson & Johnson vaccines.
Sixteen percent of the DuPage County population, or 148,000 residents, has been fully vaccinated as of March 25, and 248,000 DuPage County residents have received at least one dose of the vaccine. The number of new cases per day in the county has dropped from about 800 in December to 200 in April.
Currently, DuPage County is up to Phase 1b+ in vaccine distribution, which includes people 16-64 with high-risk medical conditions along with more essential workers. Phase 1b+ is the stage after Phase 1a (healthcare workers) and 1b (frontline workers, individuals 65 or older and incarcerated individuals). The department moved on to Phase 1b+ after 70% of people aged 65+ were vaccinated.
“Just because we moved on, it does not mean that everybody in those phases is vaccinated yet,” said Stephanie Calvillo, Public Information officer at the DuPage County Health Department.
Some students at Naperville Central have been able to receive the vaccine because they qualified for earlier phases. Lily Patterson, a senior at Naperville Central, is one such exception. She was 17 when she got the Moderna vaccine, which, at the time, was reserved for people 18 and older. She qualified to receive the vaccine so early because she works in healthcare.
“I had a prescription written by my doctor essentially giving me approval to get it,” Patterson said.
There have been some hurdles to the vaccine rollout due to earlier shortages and logistical issues. The abruptness of the virus has put healthcare workers and scientists into overdrive to search for and distribute a vaccine. As a result, this frantic push has led to very low supply and overwhelming demand.
“We see a lot of people’s frustration for those that are eligible in getting the vaccine and who’ve had a difficult time getting appointments,” Calvillo said.
Additionally, in a national CBS News poll conducted in February, a quarter of respondents said they did not want to get the vaccine. When asked why, 58% believe the vaccine still lacks testing. While most vaccines take 10-15 years to fully develop, test and study, the COVID-19 vaccine took less than a year. However, its foundations are built on prior research.
“Although it was developed quickly, there’s a reason how that was even possible,” Calvillo said. “That was because we already had years of research and effort that have gone into studying SARS, so we had some technology already built… These are safe, these are tested and we are very confident in them.”
Patterson echoes this sentiment. Working as a pharmacy technician, she’s seen the fervor to push out a vaccine and the amount of effort that goes into it.
“I understand where [these skeptics] are coming from,” she said. “[But] being in healthcare and being exposed to these different illnesses and these preventable sicknesses, getting a vaccine should not be a debate.”






John • Apr 20, 2021 at 5:01 pm
nice one jeremy